Pinpointing hydrogen isotopes in titanium hydride nanofilms
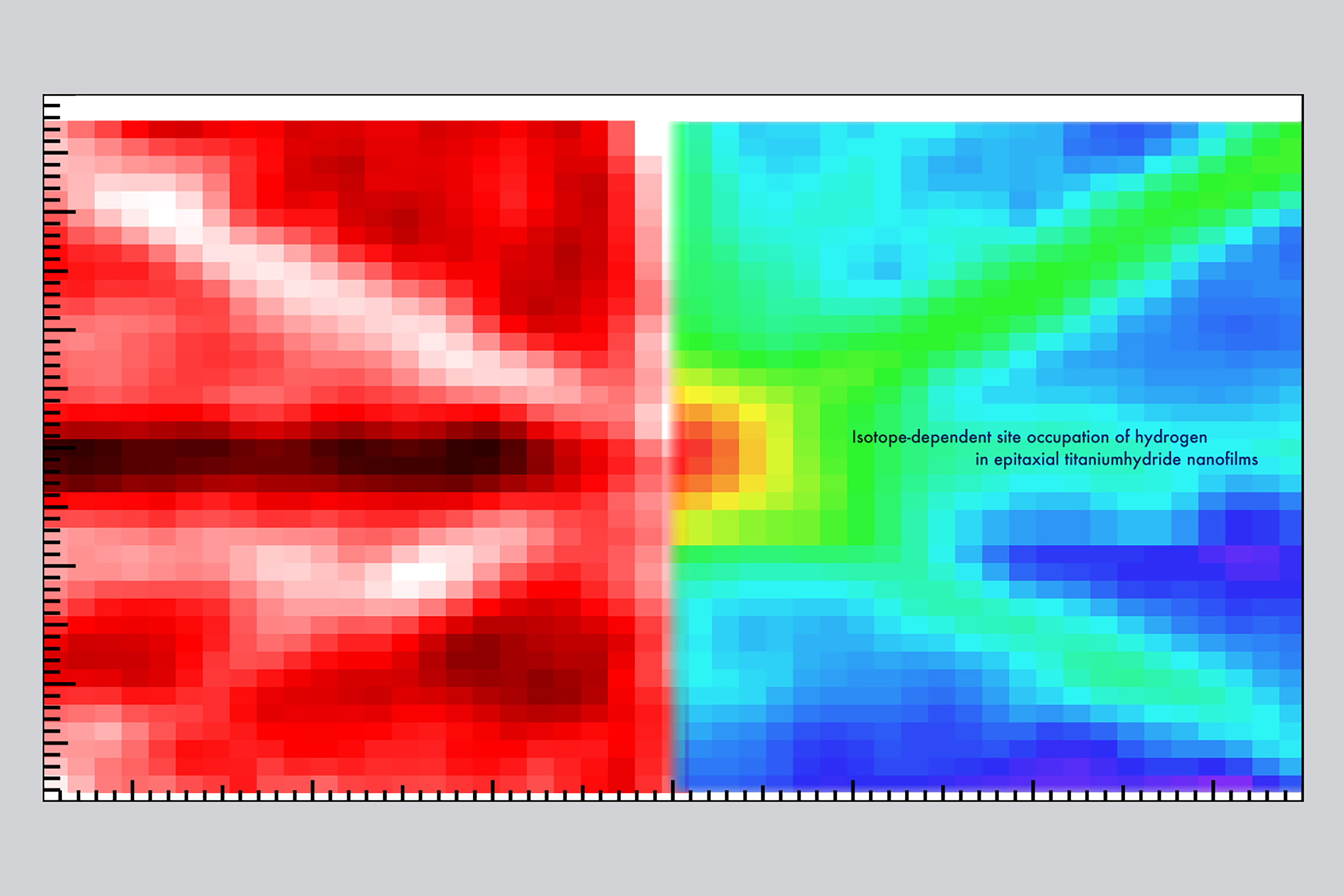
Researchers from the Institute of Industrial Science, The University of Tokyo combined nuclear reaction analysis and ion channeling to locate hydrogen and deuterium atoms in titanium hydride nanofilms. They found that 11% of the hydrogen present was at octahedral sites, with the remainder at tetrahedral sites. In comparison, deuterium was only found at tetrahedral sites. This understanding will allow control of hydride nanofilm properties to discover new hydrogen-induced phenomena.
Although it is the smallest and lightest atom, hydrogen can have a big impact by infiltrating other materials and affecting their properties, such as superconductivity and metal-insulator-transitions. Now, researchers from Japan have focused on finding an easy way to locate it in nanofilms.
In a study published recently in Nature Communications, researchers from UTokyo-IIS have reported a method for determining the location of hydrogen in nanofilms.
Because they are very small, hydrogen atoms can easily migrate into the framework of other materials. Titanium absorbs hydrogen to give titanium hydrides, making it useful for applications such as hydrogen storage.
Understanding how many hydrogen atoms are present and where exactly they are can provide the key to tuning the properties of the material. However, detecting hydrogen with commonly used techniques—such as electron probes and X-rays—is challenging because of their lack of sensitivity for the small atoms.
The researchers combined two techniques—nuclear reaction analysis (NRA) and ion channeling—to generate two-dimensional angular mapping of titanium hydride nanofilms.
“We took a close look at a TiH1.47 nanofilm,” explains lead author of the study Takahiro Ozawa. “Understanding nanofilms is useful as many hydrogen-related applications involve surface and subsurface reactions. We were able to precisely locate both the hydrogen and deuterium atoms in the nanofilm.”
All the deuterium atoms—an isotope of hydrogen with double its mass—were at locations in the titanium crystal known as tetrahedral positions. However, 11% of the hydrogen atoms present were at sites described as octahedral. Calculations showed that having this variety in the sites lowered the symmetry, which made the lattice more stable.
Because the deuterium atoms didn’t occupy octahedral sites because of nuclear quantum effects, controlling the ratio of hydrogen isotopes could be used as a means of tuning the stability and properties of nanofilms based on the intended application.
“Being able to differentiate between the two isotopes in the hydride revealed an opportunity for control,” says Katsuyuki Fukutani, senior author. “This will clearly have important practical applications for producing particular hydrogen-induced phenomena.”
The enhanced understanding of titanium hydride nanofilms is also expected to contribute to hydrogen storage, solid electrolyte, and heterogeneous catalysis applications as we move toward practical and safe green solutions for the future.
The article, “Isotope-dependent site occupation of hydrogen in epitaxial titanium hydride nanofilms,” was published in Nature Communications at DOI: 10.1038/s41467-024-53838-6
Image Credit: Katsuyuki Fukutani Laboratory

Professor Katsuyuki Fukutani


Comments
No comments yet.
Join by voting
How did you feel about the "Possible Future" depicted in this article? Vote on your expectations!
Please visit the laboratory website if you would like to learn more about this article.
Share